Acetylsalicylic acid, also known as aspirin, is an analgesic, antipyretic, and anti-inflammatory drug used for the treatment of pain, feve...
Acetylsalicylic acid, also known as aspirin, is an analgesic, antipyretic, and anti-inflammatory drug used for the treatment of pain, fever, and inflammation. The structure-activity relationship (SAR) of acetylsalicylic acid can be explored by examining the effects of modifications to its structure on its biological activity.
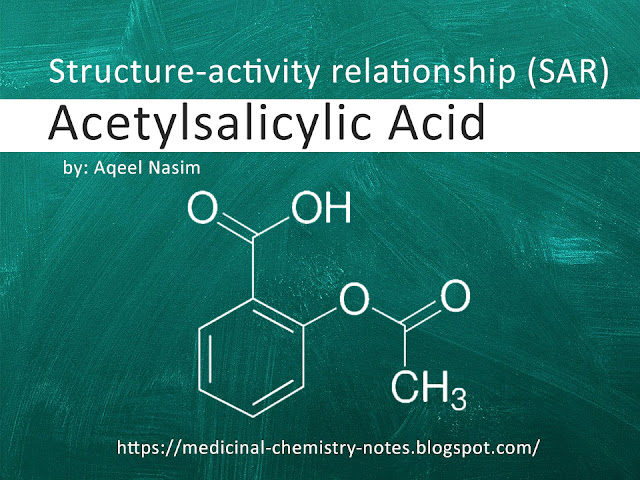
Acetyl group: Acetylsalicylic acid contains an acetyl group (-COCH3) at the 2-position of the phenolic ring. This acetyl group is important for the biological activity of the compound. Replacement of the acetyl group with other substituents, such as ethyl or propyl groups, results in a decrease in activity.
Phenolic ring: The phenolic ring in acetylsalicylic acid is important for its activity. Modification of the ring, such as substitution of the hydroxyl group with a methyl or ethyl group, results in a decrease in activity.
Carboxylic acid group: The carboxylic acid group in acetylsalicylic acid is important for its activity. Removal or modification of this group results in a decrease in activity.
Stereochemistry: The stereochemistry of acetylsalicylic acid is important for its activity. The (S)-enantiomer is more active than the (R)-enantiomer.
Lipophilicity: The lipophilicity of acetylsalicylic acid is important for its activity. Increasing the lipophilicity of the molecule through the addition of alkyl groups or the introduction of a bulky substituent at the ortho position results in an increase in activity.
Stability: The acetyl group in acetylsalicylic acid makes it more stable than salicylic acid, which is rapidly metabolized in the body. This stability is important for the long-term efficacy of the drug.
Overall, the SAR of acetylsalicylic acid suggests that the acetyl group, phenolic ring, and carboxylic acid group are essential for its activity, while modifications to the stereochemistry, lipophilicity, and stability can affect its potency.
No comments